On Thursday, December 7, a total of thirty-three students were invited to the Breazeale Nuclear Reactor at Penn State University.
Students were taken on a tour of the reactor in two separate groups, hosted by the Nuclear Engineering undergrads. During this trip, students were able to do an assortment of fun experiments to help them further understand the different forms of radiation and how they work.
Firstly, students learned about the three main types of radiation, Alpha, Beta, and Gamma. Alpha radiation is where two protons and two neutrons are bound together into a particle identical to a helium-4 nucleus, or in simpler terms, a larger particle that comes from the nucleus of an atom. Beta radiation is an electron that gets kicked out of the nucleus. More specifically, a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. Gamma radiation is a high-energy wave, penetrating form of electromagnetic radiation arising from the radioactive decay of atomic nuclei.

The tour began in the radiation lab. Students used a Geiger-Müller radiation detector (commonly called, “Geiger counters”) to test different household objects for radioactive properties. One of the items, an orange Fiestaware™ plate, was radioactive. Why was this plate radioactive? The glaze used to make it orange had uranium in it. Plates weren’t the only radioactive objects students held. Lantern mantles were tested and found radioactive because they contain thorium, which is a naturally occurring radioactive element. Thorium also has a bright white glow that makes it perfect for lanterns. Students also received items like surgical gloves, gauze, and irradiated salt that weren’t radioactive because exposing something to radioactivity doesn’t make it radioactive.
After the radiation lab, students took part in an alpha/beta/gamma exercise. Each group was given three cards with attachments of small radioactive pieces. Using a variety of materials, and their knowledge, students had to figure out what type of radiation was given off by each card attachment.

The next activity on the group’s itinerary was the cloud chambers. Petri dishes were handed out containing a felt pad that held ethyl alcohol and multiple radioactive objects. Students were asked to place their hands on top of the Petri dishes to start heating them. The purpose of doing this was to begin evaporating the ethyl alcohol within the felt pad, this created a supersaturated atmosphere. What does this mean? A supersaturated atmosphere occurs when the humidity in an area goes over 100%. When this happened inside the Petri dish, the air could no longer hold some of the ethyl alcohol as a gas. The radioactive waves from the other objects within the dish reacted with the overflow of ethyl alcohol, attracted it, and condensed it to begin creating small clouds. These small, condensed clouds looked like small trails shooting around the Petri dish.
Following that, students moved upstairs to learn about fission and fusion. There was a slideshow presentation discussing how the two processes intertwine and allow us to achieve energy. The group of students learned that fission and fusion are nuclear reactions. Fission is a process that breaks apart atoms into two parts. Fusion forces the two parts of an atom together to create a new atom. This forceful connection is the part that releases the most amount of energy.
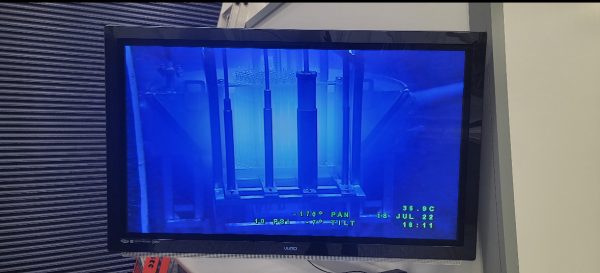
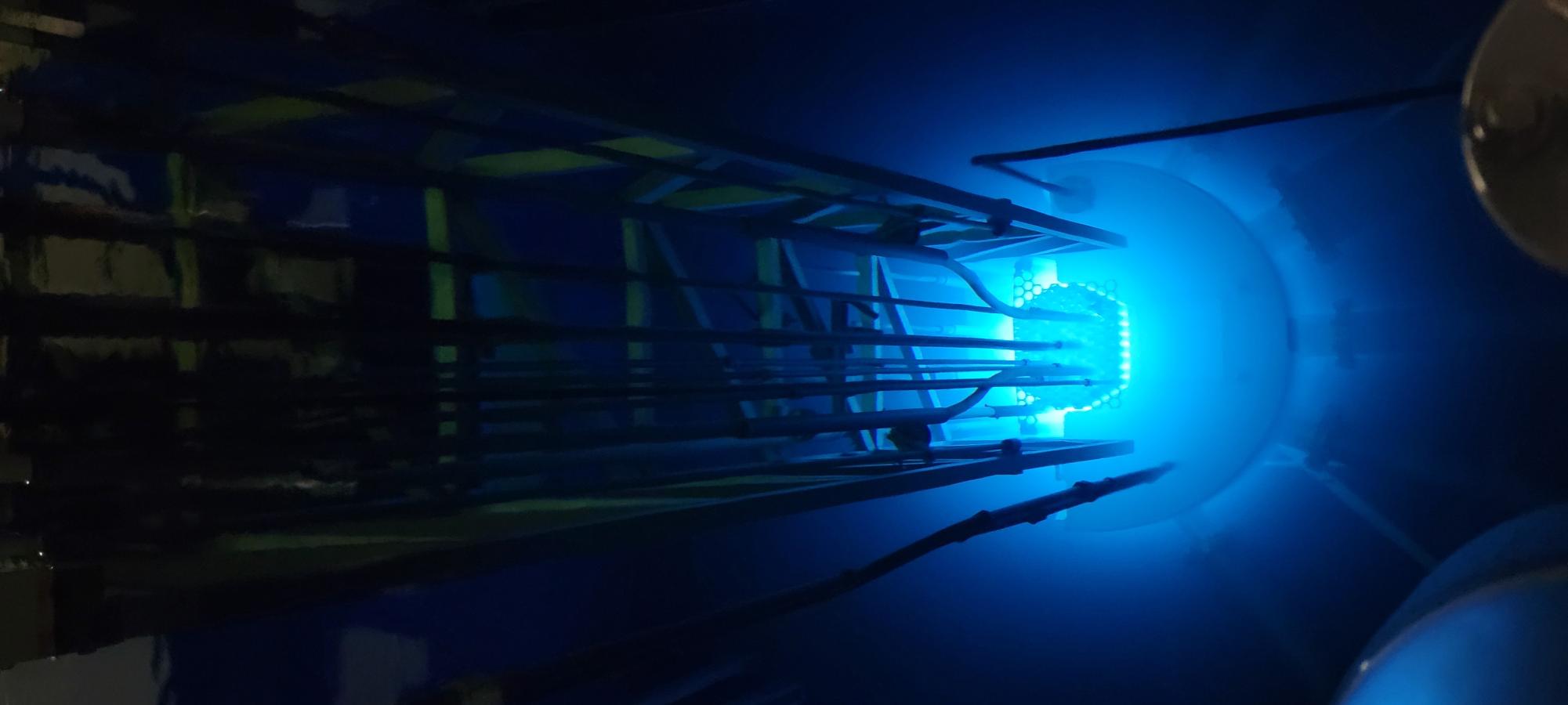
After the presentation of fission and fusion, they moved on to the reactor bay. The reactor was beneath the students and surrounded by a large pool of room-temperature water. This is because the subatomic particles from the radiation are slowed down by the hydrogen bonds significantly. These blue-hued particles, which typically travel faster than water’s speed of light, can’t harm anyone in the room observing. The subatomic particles have a blue glow because of their speed, which causes Cherenkov radiation. Zach Van Horn, the Gamma Radiation Supervisor at Penn State, compared this reaction to the shockwave energy that the sound waves give off from a sonic boom. Even being operated at only 20% of its licensed power, the reactor still gave off a strong, blue glow leaving the students in awe.
The final stop of the tour was the control room. This is where students learned about how the control rods have to be at equilibrium to allow the reactor to keep functioning properly. Lowering the rod too much will cause the reaction to absorb more neutrons than it can handle, but if it isn’t lowered enough, there aren’t enough neutrons provided to allow the cycle to continue. The perfect balance will cause neutrons to split uranium atoms and give off a stable amount of energy.
Overall, experimenting in the radiation lab, learning about fission and fusion, seeing the nuclear reactor, and visiting the control room gave students a very enjoyable and informational field trip. The students who attended would like to give their thanks to Anthony Fleury, Sharon Fredericks, Dave Prushinski, and Michael Stachowiak for organizing and chaperoning this field trip. They would also like to extend a special thank you to Zach Van Horn and Zachary Veselich for volunteering their time and spreading their extensive knowledge to the students of Greater Nanticoke Area. Field trips like this allow students to extend their learning outside the classroom. It builds knowledge; it builds interest; it builds a foundation for the future.